Aminoacids-Protein | UK-Sir-Notes | Biomolecule-2
Amino acid and Protein (Biomolecules)
 |
| Proetin |
Want to Know about Carbohydrates :
https://uksirnotes.blogspot.com/2021/09/Biomolecules-Carbohydrates-uksir.html
Want to Know about Chromosome, type of chromosome:
AMINO ACIDS
These are organic acids with Carboxylic
group (-COOH), Amino group (-NH2)
attached to α-carbon. There is also presence of a R-group and one H.
Or
Amino acids are
units/ building blocks of Proteins.
Their main contain
is C,H,O,N and S may present
General Structure:
 |
| Amino acid |
Ø Twenty Natural
amino acids are found.
Ø There is also
presence of Non-protein amino acids: Homo-arginine, Gamma-aminobutyric acid etc
Classification: (Basis- type of R- Group)
 |
| 20 natural amino acids |
1) Neutral A.A.: (with non-cyclic Hydrocarbon chain)
Ex-Glycine,
alanine, Valine, Leucine, , Isoleucine
 |
| neutral amino acids |
 |
| Neutral aa 2 |
2) Acidic A.A.: (extra carboxyl group)
Ex-Glutamic acid, Aspartic
acid, Glutamine, Asparagine
 |
| Acidic-amino-acid |
3) Basic amino acids: (additional amino group)
Ex- Arginine,
Lysine
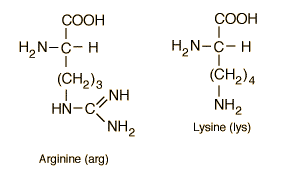 |
| Basic amino acid |
4) Alcoholic A.A.: (Alcohol or Hydroxyl group)
Ex- Serine, Threonine
 |
| Alcoholic aa |
5) Sulphur containing A.A.: (Cysteine and Methionine)
 |
| Sulphur AA |
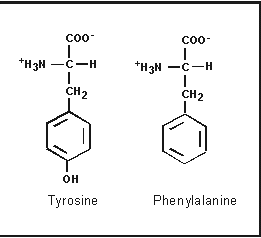
6) Aromatic Amino Acids :( Contain Cyclic structure as R group) Ex-Phenyl Alanine, Tyrosine
 |
| Aromatic |
7) Hetero Cyclic A.A.: (Nitrogen in Ring Structure)
Ex- Histidine, Proline,
Tryptophan
 |
| Heterocyclic |
New
21st and 22nd amino acids:
 |
| New-Amino-acids |
Amino acids can exit in any form of the following:
 |
| Zwitter-ion |
·
Plants
can synthesize all Amino acids, But animals cannot.
·
9
Amino Acids cant produced by Human called as Essential A.A. (Leu ,ILeu, Val,
Tryp, Phy Ala, Lys ,Thr and Met)
Function:
·
Basic units of Proteins.
·
Storage of Nitrogen in form of Amides.
·
Production of various Vitamins and Hormones
·
Production of antibiotics etc.
PROTEIN: (Proteios Gk- First of formost)
Proteins
are nothing but poly peptide chains of amino acids.
Or
Proteins
are large sized hetero polymeric macromolecules having one or more poly
peptides.
Protein
term coined by – Berzelius (1837) and Mulder (1838)
Ø Single
peptide chain can contain minimum of 50 AA
Ø Proteins
can be 50% of the dry weight of the body.
Ø In
Bacterial cell- 1000-2000 type of protein.
Ø 20
natural amino acids form protein.
Ø A.A.s
contain peptide bonds between Amino gr and Carboxyl gr.
Ø A
protein with 2 or more peptides – multimer or multimeric protein (Ex- Heamoglobin)
Level of structural organization:
4
levels of organization
1) Primary structure:
Ø Basic
structure of a protein
Ø Contain
only amino acids
Ø Formed
by translation process
Ø Distance
between two peptide bonds- 0.35nm
Ø Extended
between N- terminal and C- terminal.
 |
| Primary protein |
2) Secondary Structure:
Ø If
primary protein develop various interactions, form secondary structure.
Ø They
develop 3 type of structure
Ø α – helix – spirally coiled secondary structure,
right handed coiling, H-bond between
Amino and carboxyl group , single strand
or multiple strand, (Ex- keratin, myosin, fibrin)
 |
| secondary protein |
Ø β – Pleated- 2 or more poly peptide
inter connected by H-bond, a sheet or flat
structure, may be parallel (keratin) or anti parallel (fibroin of silk)
Ø Collagen
helix- most abundant protein in body, explained by Ramachandran (1954) about
the presence of 3 strand coiling.
 |
| Collagen |
3) Tertiary:
Ø Further
bending and folding
Ø Active
site comes to surface
Ø Stabilized
by various bonds like- H- bond, Ionic interaction, van der waal’s interaction,
hydrophobic interaction, covalent bond, di-sulphide bond
Ø Sometime
temp, radiation, pH etc cause degradation of Tertiary structure.
 |
| Tertiary Protein |
4) Quaternary
Structure:
Ø Multimeric
protein found in single structure.
Ø Mostly
act as the subunits of a large functional protein
Ø Heamoglobin-
2α and 2 β protein subunits
 |
| Haemoglobin |
Types:
On
the basis of Shape
1)
Fibrous:
Ø Thread
like, single or in group secondary structure
Ø Mostly
structural, non enzymatic In nature
Ø Insoluble
in water
Ø Ex
– keratin of hair
2)
Globular:
Ø Rounded
or globular
Ø Mostly
Tertiary or quaternary in structure
Ø May
be enzymatic or not, but mostly soluble in water
Ø Some
don’t coagulate by heat- Histone
Ø Ex-
Albumin, serum globulin, glutelins etc.
PROPERTIES:
Ø Due
to specific arrangement- protein show large diversity
Ø Protein
show specificity i.e. specific protein in specific species of organism
Ø They
have large mol. Wt- 4500 to 46,00,000
Ø Present
in colloidal form
Ø Some
proteins show reactivity – enzymes, anti bodies, toxins etc.
Ø Cell
membrane don’t allow the permeation (infusion or diffusion) of protein, needs
exo or endocytosis.
Ø Amphoteric
in nature
Ø May
denature their 3D structure.
FUNCTION:
Ø Protective
in Nature- Keratin, fibroin etc.
Ø Defense
mechanism-immunoglobulin, antibodies, toxins etc.
Ø Toxin
production- snake venom, ricin of castor, bacterial toxin etc.
Ø Structural
protein- 50% dry weight of protoplast
Ø Formation
of microtubules- cilia, flagella, basal bodies etc.
Ø Sieve
tube P-protein formation
Ø More
than 2000 type of enzyme formation
Ø Formation
of carrier protein- myoglobin of muscle
Ø For
storage- casein, Ovalbumin, etc.
Ø Form
protein buffer, hormones, receptors etc.
Formation of antibodies, visual pigments (rhodopsin, iodopsin) blood clotting pigment (fibrinogen, thrombin)
Want to Know about Carbohydrates :
https://uksirnotes.blogspot.com/2021/09/Biomolecules-Carbohydrates-uksir.html
Want to Know about Chromosome, type of chromosome:
That's all about Cell cycle-Mitosis and Meiosis. Feel free to ask doubts in comment section.. UKsir



No comments