Plant-Water-Transport | uksir-notes |Plant- Physiology
Plant Physiology:
Transport In Plants:
 |
| water Transport |
-
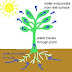
The transportation may start from root (Water and mineral) or
from Leaves (Food).
-
The transporting tissue is Xylem and phloem.
-
Plants also need Transpiration and Guttation like process for
transport of water.
Type of Transportation:
-
It is of 3 type in Plants :
-
I) Cellular Transport – With in a cell
-
Ii) Short distance Transport-
One cell to another
-
Iii) Long distance transport- Water and sugar transport
through Xylem and Phloem.
Cellular Transport:
-
In a cell, as the Plasma membrane is selectively permeable,
there are various type of transport system can be seen. They are of following
types:
Membrane Transport:
 |
| Transport Type |
As the Plasma membrane is selectively permeable, there are various type of transport system can be seen. They are of following types:
1. Passive Transport:
- Do not need any kind of energy for transport of material.
- Mostly transport occurs from high conc. Area to low conc. Area.
- Transport mainly dependent on simple diffusion or Osmosis process.
- Diffusion occurs directly or through the Channel and Carrier proteins(Facilitated diffusion)
- Neutral Solutes or Lipid soluble substances can directly pass through membrane.
- There are some open channel protein and Ion channels for facilitated diffusion.
-
Presence of Aqua porins for water transport.
 |
| Passive Transport |
2. Active Transport:
- In Active transport there is use of Energy.
- Here the transport of material occurs from lower conc. To Higher conc. i.e. against the Osmosis law.
- Active transport is of two type
- A)Primary active transport: Direct use of ATP as energy source
- B)Secondary Active transport: ATP is not used directly. Other sources for energy.
- Primary Active transport- Example- H+ ATpase Present in Mitochondria, Calcium pump ATpase in RBC and Muscle cells.
- Secondary Active transport mostly done by various carrier proteins, also called as pumps.
 |
| Types |
- Pumps are of 3 types- Uniport (1 molecule to one direction at a time )
Symport (2 Molecule transport to the same direction)
Antiport (2 molecule transport to opposite direction)
- Uniport Pumps – K+/potassium Pump, Cl- pump etc.
- Antiport Pump - Sodium Potassium Pump, Na + ca2+ exchanger in plasma membrane etc.
- Symport Pumps - K+ H+ pump symporter in root, Na+ Cl – symporter, K+ Cl – symporter loop of henle.
3. Bulk Transport:
- When cell need material in Group/bulk/in Large number, it perform bulk transport.
- It is of Two Type-
- A) Endo Cytosis: Inner ward transport of material with help of Carrier Vesicle.
- B) Exo Cytosis: Outer ward transport of material with help of Carrier Vesicle.
- Endo Cytosis is either -
- i) Phago Cytosis- Cell eating or ii) Pino Cytosis/ Poto Cytosis- Cell Drinking
- Sometime Receptor / Ligand mediated endocytosis can be seen.
 |
| Bulk transport |
Diffusion:
-
The movement of particles from their higher conc to their
lower conc, it is called as diffusion.
-
The particles may be Gas, liquid or solid.
-
They maintain equllibrium through diffusion.
-
Generally diffusion occurs due to – Particle’s kinetic energy and difference in
conc. Of particular areas.
Diffusion pressure: (DP)
-
Due to diffusion of particles a pressure is developed called
as the DP.
-
Maximum DP is of Pure Water : ~1336.40 Atm at 200C
-
It decraes when solute is added.
Imbibition :
-
When a solid particle absorb water but donot form solution, its
called as imbibition.
-
The water absorbtion may increase their size.
-
Imbibants: the solid particle which absorb water
(Hydrophillic in nature).
-
Imbibate: The liquid which is absorbed.
-
Due to imbibtion wetting heat may generate, and the
imbibition pressure is really high.
-
Ex: Wooden door swell in rainy season, seed swell up before
germination etc.
Osmosis:
-
Osmosis process was
discovered by Nollet (1748)
Definetion:
 |
| osmosis |
-
Osmosis may be defined as the flow of solvent or water
molecule from less conc. Solution area to high conc. Solution Through a semipermeable
membrane.
-
Or
-
Osmosis is the flow of solvent from its high conc. To
its lower conc. Area through a semipermeable
membrane.
-
Or
-
When water pass from its high water potential area to its
lower water potential area is called as the Osmosis process.
-
Both pressure gradient and conc. Gradient decide the
direction and rate of osmosis.
Osmotic Conc.:
-
It is the amount of dissolved solute in solution per unit
volume.
-
It can be of 3 types:
-
Hypotonic solution: if osmotic conc. Is lower then the cell
sap or another solution.
-
Hyper tonic Solution: If osmotic conc is higher then the cell
sap or another solution.
-
Isotonic Solution: If osmotic conc. Is equal to the cell sap conc.
 |
| solution-type |
Osmosis Types:
-
Generally of 2 type-according to water movement.
-
Ex-osmosis : When water comes out of the cell due to placing
it in Hypertonic solution, its called ex-osmosis. Due to ex-osmosis cell loose
turgidity and become flaccid.
-
Endosmsis : When water is absorbed by cell due to placing it
in Hypotonic solution, then its called as endosmosis. In this case cell become
more turgid.
Turgid
cell:
 |
| turgid-cell |
-
When a cell attains, its maximum size due to fully hydrated
condition, it is called as Turgid cell.
-
Now this property is called as Turgidity.
-
Here the pressure developed by cell membrane against cell
wall is called as Turgor Pressure (TP).
Osmotic pressure: (OP)
-
When a solution is separated by one semipermeable membrane
from its pure solvent (water), then the solution develops a pressure – called
as osmotic pressure.
-
OP is directly proportional to the Conc. Of the solution and
the temperature.
-
It also depends on the solute particle (Ionization and
hydration).
-
OP of plant generally varies from- 4 to 5 Atm.
Significance:
-
Help in absorption of water by cell.
-
Help in maintaining the turgidity of Cell.
-
Help in movement of plant parts.
-
Help in opening and closing of stomata.
-
Some time provide resistant to cold stress.
Plasmolysis:
-
Due to excess exosmosis in cell, cytoplasm become dehydrated.
-
Cytoplasm shrink from the cell wall, become jelly like
semisolid condition.
-
Thus due to exosmosis, shrinkage of protoplast is called as Plasmolysis
process.
The cell show plasmolysis process is called as Plasmolysed cell.
 |
| plasmolysis |
-
It may be of 3 stages:
-
Limiting Plasmolysis:
starting stage where water loss starts, vacuole and cytoplasm starts
shrinking. (Here TP= 0)
-
Incipient Plasmolysis: Due to continuous exosmosis, cytoplasm
shrinks. (Here TP= less -ve)
- Evident plasmolysis: Last stage of shrinkage, protoplast become rounded.
(Here TP = extremely -ve). A cell can’t survive in this
condition.
-
De-plasmolysis:
when a plasmolysed cell placed in water and again come to its original
size, by getting hydrated.
Plant water relationship:
As
water content in a plant cell is 75- 95%, there is a plant water relationship
develops. To define this we use dome term like- Water potential, pressure
potential, solute potential, matric potential etc.
Chemical potential:(ᴪ)
-
According to thermodynamics- free energy represents the
potential of performing work.
-
So the quantitative expression of free energy present in any chemical in a
system is called as Chemical potential.
-
It’s a relative quantity.
-
Larger the chemical potential = greater chance of chemical
reaction.
-
Chemical potential Unit is = energy in per mole
of substance (J/ mole)
Osmotic / Solute Potential: (ᴪs)
-
It is the potential of water molecule to move from less conc.
Solution to high conc. Solution through a semipermiable membrane.
-
Osmotic potential decrease
due to addition of solute in pure water.
-
There for osmotic potential always carry a –ve sign.
-
Formula:
-
ᴪs = C×R×T
-
Here C= conc. Of solute in mole/L
-
R = Universal gas constant (0.083)
-
T= Temp in absolute degree.
-
OP is less –ve at 00C and more –ve in warmer
solution.
-
This result the flow of water from colder to warmer condition.
Turgor Pressure (ᴪp):
-
The pressure of cell membrane or protoplast against cell wall
is called Turgor pressure.
-
Also called Hydrostatic pressure or Pressure potential. (ᴪp)
-
Normally in a normal condition cell Wall pressure and Turgor
pressure are almost equal.
-
TP = WP
Metric Potential (ᴪm):
-
Expression of water absorption by matrix, colloidal particle
or surface in plant cell.
-
Macrix potential show almost negligible value, which do not
considered in calculation.
-
Also called as
Chemical potential of water.
-
Or
-
It’s the difference between the free energy present in
solution to that of pure water.
-
Water potential of Pure water is maximum i.e. 0 Mpa. (1 Mpa =
10 bars.)
-
Water potential of solution is –ve as solute is added.
-
Water potential formula
-
ᴪw = ᴪs + ᴪp
+ ᴪm
-
Here ᴪw = water potential
ᴪs = solute potential, ᴪp
= pressure potential, ᴪm = matric potential.
But ᴪm is negligible
So ᴪw =ᴪs + ᴪp
Diffusion Pressure Deficit (DPD):
-
I is the difference between diffusion pressure od solution to
its pure solvent. (at particular temp, and pressure.)
-
The term was coined by Meyer (1938).
-
It determine the direction of water movement. (Water move
from High DPD to Low DPD.)
-
DPD have +ve sign.
-
DPD is directly related to conc. Of solution. (DPD α Conc. Of solution)
-
DPD is the measure of ability of cell to absorb water, therefore
also called as Suction pressure (SP).
DPD/ SP = OP – WP
But, WP =
TP (in turgid cell)
S0, DPD =
OP – TP
In fully turgid cell :
OP =TP, So, DPD = 0 (Zero)
In fully
Plasmolyzed cell : TP = 0, So,
DPD = OP /
SP = OP
***
Thank you for visiting.. feel free for asking doubt in comment session.... UK Sir


No comments